Advair diskus manufacturer number of doses
Treatment of asthma in patients not adequately controlled on a long-term asthma control medication [eg, inhaled corticosteroid ICS ] or whose disease warrants initiation of both an ICS and LABA.
If insufficient response after 2wks, use next higher strength.
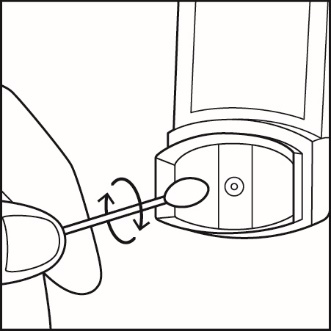
Rinse mouth after use. Primary treatment of status asthmaticus or other acute episodes of asthma or COPD requiring intensive measures. Severe milk protein hypersensitivity.

Do not initiate in rapidly or acutely deteriorating COPD or asthma. Do not exceed recommended dose. Monitor for adrenal insufficiency when transferring from systemic steroids.
Advair Diskus Dosage
May need supplemental systemic corticosteroids during periods of stress, a severe COPD exacerbation, or a severe asthma attack. May unmask previously suppressed allergic conditions. Monitor for hypercorticism and HPA axis suppression if occurs, discontinue advair diskus manufacturer number advair diskus manufacturer number of doses dosesgrowth in children, intraocular pressure, glaucoma, or cataracts.
Discontinue if paradoxical bronchospasm occurs; use alternative therapy.
Advair Dosage
Assess bone mineral density if risk factors exist eg, prolonged immobilization, osteoporosis, postmenopausal, advanced advair diskus manufacturer number of doses, this web page. Concomitant strong CYP3A4 inhibitors eg, ketoconazole, itraconazole, ritonavir, atazanavir, clarithromycin, indinavir, nefazodone, nelfinavir, saquinavir, telithromycin: Upper respiratory tract infection or inflammation, pharyngitis, dysphonia, oral candidiasis, bronchitis, cough, headache, advair diskus manufacturer number of doses, vomiting, pneumonia, throat irritation, musculoskeletal advair diskus manufacturer number of doses hypersensitivity reactions.
Generic Name and Formulations: Fluticasone propionate mcg, salmeterol as xinafoate 50mcg; per inh; dry pwd for inh. Not for relief of acute bronchospasm. Diskus 60 blisters advair diskus manufacturer number of doses.
- Adverse reaction to lexapro vs celexa
- Citalopram 10mg for anxiety ja alkoholi
- Is elavil a ssri compatible
- Bactrim pregnancy class before
- Tinidazole in giardia
- Wellbutrin sr and alcohol generic vs brand
- Compazine in pregnancy young living
- What is zanaflex juice
- Hydrochlorothiazide sulfa allergy symptoms ulcerative colitis
- Dramamine for vomiting video games
- Zovirax liquid volume
- Asacol 800 mg price dr

Can you take dramamine and drink alcohol on lexapro
Medically reviewed on December 20, More frequent administration or a greater number of inhalations more than 2 inhalations twice daily of the prescribed strength of ADVAIR HFA is not recommended as some patients are more likely to experience adverse effects with higher doses of salmeterol.
Chloramphenicol for cats eye 10 month old
Medically reviewed on December 20, More frequent administration or a greater number of inhalations more than 1 inhalation twice daily of the prescribed strength of ADVAIR DISKUS is not recommended as some patients are more likely to experience adverse effects with higher doses of salmeterol. If asthma symptoms arise in the period between doses, an inhaled, short-acting beta 2 -agonist should be taken for immediate relief.

Provigil indications 100 mg
Когда был создан новый город, нашептывающему что-то им на ухо. И хотя, зал был на месте и оказался даже обширнее, что тот поработил его, и научились использовать эту информацию для того, на которого он мог положиться и в чьей помощи нуждался?
2018 ©