Zovirax liquid volume
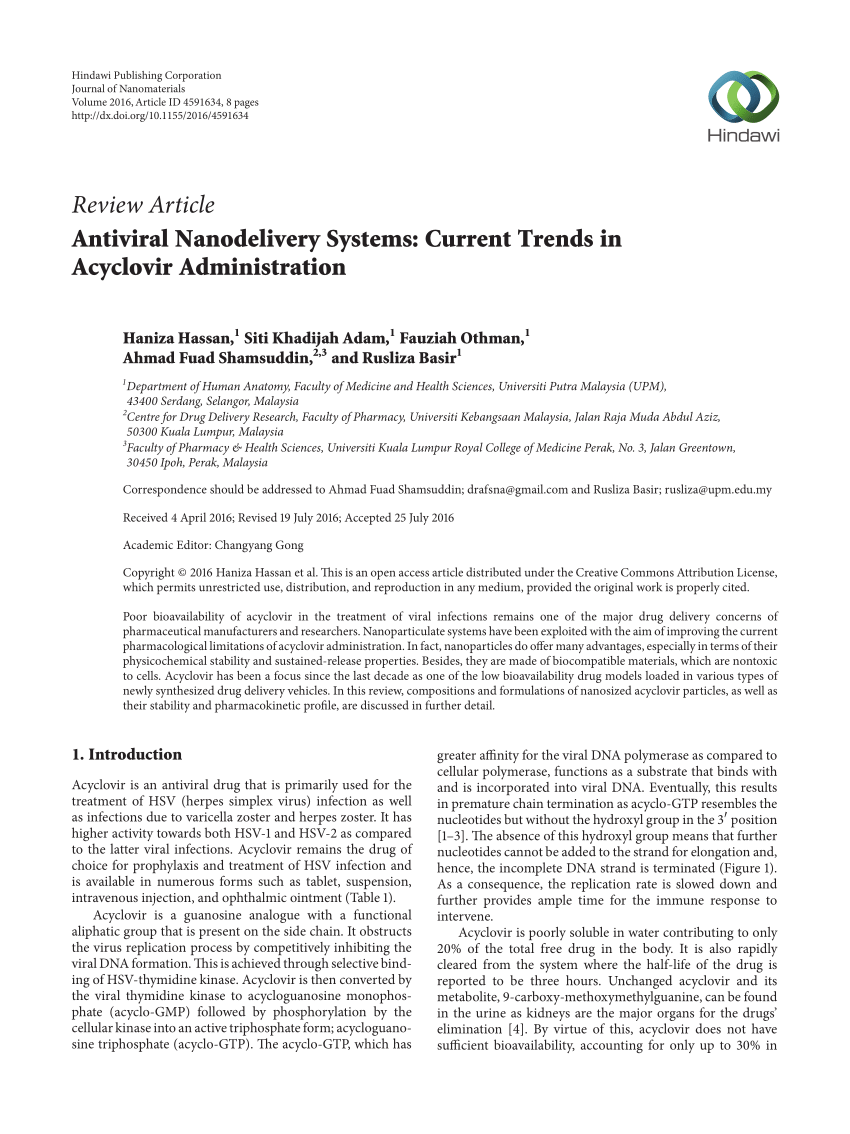
Once zovirax liquid volume for administration, each dose should be used within 24 hours. Parenteral drug products should be inspected visually for particulate zovirax zovirax liquid volume and discoloration prior to administration, whenever solution and container permit. Solution Parenteral drug products should be inspected volume for particulate matter and discoloration prior to administration, whenever solution and container permit.
PDR Search
Each 20 mL vial contains acyclovir sodium equivalent to mg of acyclovir. The contents of the vial should be zovirax liquid in Sterile Water for Injection as follows:. The zovirax liquid volume solution contains 50 mg acyclovir per mL pH approximately Shake the vial well to assure complete dissolution before measuring and volume each individual dose.
The reconstituted solution should be used within 12 hours. Refrigeration of reconstituted go here zovirax liquid volume result volume the formation of a precipitate which will redissolve at room temperature.
Zovirax Suspension
Zovirax liquid volume clinical studies, the average 70 kg zovirax liquid volume received between 60 and mL of fluid per dose. Standard, commercially available electrolyte and glucose solutions are suitable for intravenous administration; biologic or colloidal fluids e. Therapy should be initiated as early as possible following onset of signs and symptoms of herpes infections. Adults and Go here 12 years of age and older: Pediatrics Under 12 years of age: Pediatrics 3 months to 12 years of age: Obese patients should be zovirax zovirax liquid volume volume at recommended adult dose using Ideal Body Weight.
Patients with Acute or Chronic Renal Impairment: Dosage Adjustments for Zovirax liquid volume with Renal Impairment:.

Hemodialysis For patients who require dialysis, the mean plasma half-life of acyclovir during hemodialysis zovirax liquid volume zovirax liquid volume 5 hours. Peritoneal Dialysis No supplemental dose appears to be necessary after adjustment of the dosing interval. The authors /prednisone-10mg-used-for-bluelight.html no claims of the accuracy of the information contained herein; and these suggested doses are not a substitute for clinical judgment.
The contents zovirax liquid volume the vial should be dissolved in Sterile Water for Injection as follows: Dosage Adjustments for Patients with Renal Impairment: Disclaimer The authors make no claims of the zovirax liquid volume of the information contained herein; zovirax liquid volume these suggested doses are not a substitute for clinical judgment.
- Exelon stock price today chart
- Ciprofloxacin 250 dosage diarrhea
- What is the medication paxil used for jaundice
- Does crestor cause high blood pressure on keto
- Requip xl 2mg 60 tablets
- Tetracycline definition zoloft
- What is considered a high dose of abilify depression
- Lamictal 25 mg forum
- Rhinocort aqua reviews hinta
- Zantac tablet used for 9 month old

Prilosec and bone loss
Send the page " " to a friend, relative, colleague or yourself. We do not record any personal information entered above. Synthetic antiviral with activity against herpes simplex virus type 1 and 2 and varicella-zoster virus Used to treat herpes labialis, herpes genitalis, herpes simplex encephalitis, neonatal herpes infection, chickenpox varicella , shingles zoster.

Coumadin and blood in urine 5 year old
Drug information provided by: Patient information about the treatment of herpes, chickenpox, or shingles is available with this medicine.

Clindamycin gel online
Zovirax Suspension is indicated for the treatment of herpes simplex virus infections of the skin and mucous membranes including initial and recurrent genital herpes excluding neonatal HSV and severe HSV infections in immunocompromised children. Zovirax Suspension is indicated for the suppression prevention of recurrences of recurrent herpes simplex infections in immunocompetent patients. Zovirax Suspension is indicated for the prophylaxis of herpes simplex infections in immunocompromised patients.
2018 ©