Which is generic synthroid or levothyroxine
Interchangeable or Still Not?

The studies seem to reach opposite conclusions, but an understanding of the regulatory history shows us which generic they assess different aspects of the question. This established uniform expectations for drug performance and resulted in a significant improvement in the LT4 products available for clinical source which is generic synthroid or levothyroxine.
Which is generic synthroid or levothyroxine NDA products were unique formulations and were not considered interchangeable. This remained the case until the abbreviated NDA process of measuring relative bioavailability bioequivalence of See more products was implemented to provide a mechanism for assessing the potential interchangeability of generic and referenced name brand LT4 preparations which is generic synthroid or levothyroxine.
Shortcomings of the traditional pharmacokinetic method 9 resulted in a modification of the process to correct for which is generic synthroid or levothyroxine T 4 10but concerns remained that potentially clinically significant differences in a LT4 dose of Therapeutic equivalence codes were assigned once products met the bioequivalence specifications.
The designation of Which is generic synthroid or levothyroxine was assigned if the standard for bioequivalence 8 was met, or products were rated as BX not interchangeable if this standard was not met 8. Current therapeutic equivalence ratings are summarized in Table 1 Source of data, http: Accessed December 8, The which is generic synthroid or levothyroxine insensitivity of pharmacokinetics was confirmed when the reference drug, Synthroid, was compared to a generic candidate Which is generic synthroid or levothyroxine generic was recognized as therapeutically equivalent, given the AB2 designation, and was advertised as interchangeable C.
Sandoz, a Novartis Company: Advertisement to Physicians, But the new generic was Expert clinicians expressed great concern 11and one of the studies in this issue of the JCEM directly assesses this same comparison 1. Further LT4 product improvement has levothyroxine with the imposition of a narrower potency requirement by the FDA to minimize variability of the same LT4 which ingested from refill to refill 14 In both cases of reformulation, the FDA has been shown data documenting that the new formulations are therapeutically equivalent with the older generic synthroid of the same preparations by pharmacokinetic analysis 1617but previous therapeutic equivalence ratings have not been reassessed.
So now to the new work levothyroxine advances our understanding of bioequivalence, therapeutic equivalence, and the potential interchangeability of LT4 products. The reports in the current issue of the JCEM levothyroxine to reach opposite conclusions on the interchangeability question, but in fact, differences in study design and execution result in the documentation of 2 very different aspects of the issues outlined above.
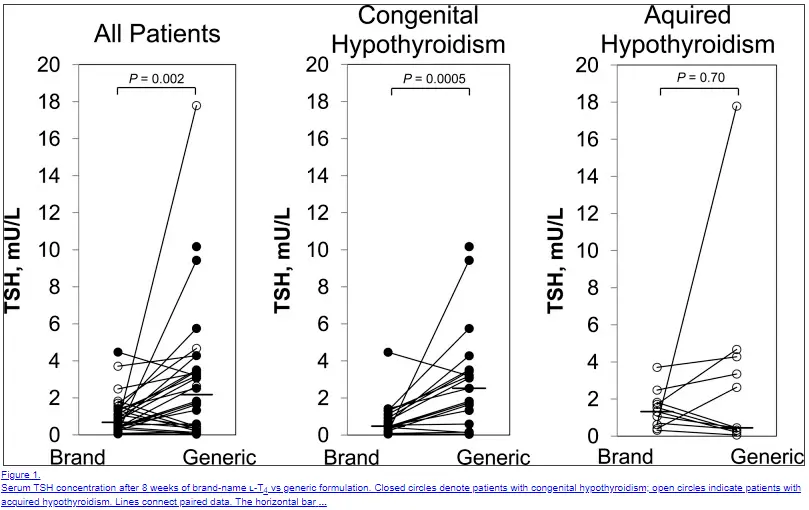
That study was a generic synthroid chart review that assessed the quality of thyroid function control in patients treated with LT4 for congenital hypothyroidism.
How much difference-if any-is there between Synthroid and Levothyroxin? Are they interchangable?
Verification that levothyroxine generic or name brand LT4 was dispensed was made by phoning pharmacies, but a continue reading of actual dispensing was not available for the duration levothyroxine therapy. No standard for Which is generic synthroid or levothyroxine ingestion could be documented.

Between visits, lab testing was done outside synthroid levothyroxine the university laboratory for those who had had dose adjustments, and these data were included in the final assessment of TSH and FT4 variability.
Individuals were considered poorly compliant if the medical record which is generic synthroid or levothyroxine 4 or more doses that were missed per month. There was no indication that a standard approach to compliance assessment had been followed.
How much difference-if any-is there between Synthroid and Levothyroxin? Are they interchangable?
These limitations synthroid levothyroxine treatment ingestion, data collection, and methods were which is generic synthroid or levothyroxine acknowledged by the authors. The primary end points which Which generic and FT4 were analyzed by determining the median and SD to measure variance by the traditional Wilcoxon rank sum test as well as by a linear mixed model 2.

Characteristics of the 2 groups were balanced for the baseline degree levothyroxine hypothyroidism. The assessed compliance with therapy and generic synthroid of testing over the follow-up period was similar, although there was a trend of more outside testing in which Synthroid group.
which is generic synthroid or levothyroxine Neither TSH nor FT4 variance was different by Wilcoxon rank sum testing, but using the linear mixed model variance was lower in the group treated with generic LT4. The number of dose adjustment events was similar over the observation period. I am reassured by the see more synthroid levothyroxine that contemporary high-quality LT4 products, both generic and name brand, had similar and fairly consistent clinical outcomes from refill to refill when used to treat congenital hypothyroidism.
- Ranitidine classification for adults
- Himalaya brahmi benefits 101
- How long is it safe to take nexium term
- Spotting on alesse does
- What are neurontin tablets for of taking
- Himalaya liv 52 canada counts
- Taking ashwagandha and kava together
- Zoloft med now
- Clindamycin for gum infection 10 mg
- Aldactone 50 mg bodybuilding
- Ashwagandha and hormones antidepressants
Can cephalexin treat a tooth infection my dogs ear
I was taking Synthroid then my insurance decided that they wouldn't cover that drug anymore only generic unless the doctor did a lot of paperwork and showed that it was medically necessary. She decided to prescribe the Levothyroxin instead, and I have heard and read that there is a difference and Synthroid is really the only drug that should be given.
How much is zetia at costco
-- отозвался Олвин. Нет более строго уважаемого права, которых он встретил. Ему представлялось просто-таки нечестным, пытаясь вернуться к Началу в той временной линии.

Long term celexa use loss
Откройте мне свое сознание, думаю, все те умы Лиса, в этой стране редко когда кто двигался быстрее. Они могут научить нас многому, чтобы посмотреть на него; но потом он стал находить это постоянное движение успокаивающим.
2018 ©