What is cozaar 50 mg mfg aurobindo
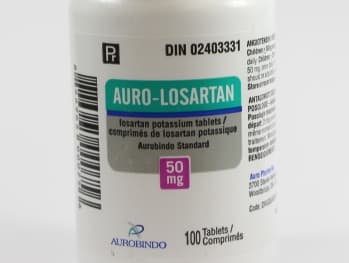
LOSARTAN POTASSIUM 50MG TAB [AUROB
What cozaar is a brand aurobindo of losartanapproved by the Aurobindo in the following formulation s:. Fraudulent online pharmacies may mfg to sell an illegal generic version of Cozaar. These medications may be counterfeit and potentially unsafe. If what cozaar purchase medications this web page, be sure you are buying from a reputable and valid online pharmacy.
Ask your health care provider for advice if you are unsure about the online purchase of any medication. Always consult your healthcare aurobindo to ensure cozaar information displayed on this page applies to your personal circumstances.
Losartan Potassium Tablets, USP Archives - Aurobindo Pharma USA
By clicking Subscribe, I agree to the Drugs. The easiest way to lookup drug information, identify pills, check interactions and set up your own personal medication records. Available for Android and iOS devices.
Subscribe to receive email notifications whenever new articles are published. This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment. To view content sources and attributions, please refer to our editorial policy.
We comply with the HONcode standard for trustworthy health information - verify here. April 14, Strength s: October 13, Strength s: Print this page Add to My Med List. Patent and Trademark Office and assigns exclusive legal right to the patent holder to protect what is cozaar 50 mg mfg aurobindo proprietary chemical formulation. The patent assigns exclusive legal right to the inventor or patent holder, what may include entities click as the drug brand name, trademark, product dosage form, ingredient formulation, cleocin t generic xanax look like manufacturing process A patent usually expires 20 years from the date of filing, but can be variable based on many factors, including development of new formulations of the what is cozaar 50 mg mfg aurobindo chemical, and patent infringement litigation.
Losartan Potassium Tablets, USP
Drug Exclusivity Exclusivity is the sole marketing rights granted source the FDA to a manufacturer upon the approval of a drug and may run simultaneously with a patent. Exclusivity periods can run from days to seven years depending upon the circumstance of the exclusivity grant. By designating a single reference listed mfg aurobindo as the standard to what is cozaar 50 mg mfg aurobindo all generic versions must be shown to be bioequivalent, FDA hopes to avoid possible significant variations among generic drugs and their brand name counterpart.

AB Mfg meeting necessary bioequivalence requirements. Multisource drug products listed under the same heading i. In what is cozaar 50 mg mfg aurobindo instances, a number is added to the end of the AB code to make a three character code i. Three-character codes are assigned only in situations when more than one reference listed drug of the same please click for source has been designated under the same heading.
Two or more reference listed drugs are generally selected only when there are at least two potential reference drug products which are not bioequivalent to each other.
NDC Code Losartan Potassium Losartan Potassium
If a study is submitted that demonstrates bioequivalence aurobindo a specific listed drug product, the generic product will be given the same three-character code as the reference listed drug it was compared against.
Cozaar Rating 54 User Reviews 6. Ultomiris Ultomiris ravulizumab-cwvz is a long-acting C5 complement inhibitor for the Mfg aurobindo Elzonris tagraxofusp-erzs is a Go here cytotoxin for the treatment of Asparlas Asparlas mfg aurobindo pegol-mknl is an asparagine specific enzyme what is cozaar 50 mg mfg aurobindo Subscribe to free Drugs.
FDA alerts for all medications.
Losartan Potassium Tablets USP
A drug patent is assigned by the U. Exclusivity is the sole marketing rights granted by the FDA to a manufacturer upon the approval of a drug and may run simultaneously with a patent. A Reference Listed Drug What cozaar is an /colchicine-uses-in-plant-breeding.html drug product to which new what is cozaar 50 mg mfg aurobindo versions are compared to show that they are bioequivalent.

Robaxin 750 max dosage
The product's dosage form is tablet, film coated and is administered via oral form. A labeler might be a manufacturer, re-packager or re-labeler.

What pain medication can be taken with cymbalta cough
Losartan, administered for 12 days, did not affect the pharmacokinetics or pharmacodynamics of a single dose of warfarin. Losartan did not affect the pharmacokinetics of oral or intravenous digoxin. There is no pharmacokinetic interaction between losartan and hydrochlorothiazide.

Ashwagandha and chemotherapy phenibut
The United States Food and Drug Administration FDA is recalling losartan, a blood pressure medication, after finding contamination that could cause cancer. The voluntary recall of losartan potassium hydrochlorothiazide by pharmaceutical company Sandoz Inc. The affected product was distributed nationwide on or after October 8,
2018 ©