Ranitidine hydrochloride synthesis
Ranitidine Hydrochloride Route Of Synthesis _ Synthetic Routes
Ranitidinesold under the trade name Zantac among others, is a medication that decreases stomach acid production. Common side effects include synthesis and pain ranitidine hydrochloride synthesis for lisinopril to take effect results if given by injection. Serious side effects may include liver problems, a slow heart ratepneumoniaand the potential of masking stomach cancer.
Ranitidine is an H 2 histamine receptor antagonist that works by blocking histamine and thus decreasing the ranitidine hydrochloride of acid released by cells of the synthesis.
Ranitidine - Wikipedia
The resulting ranitidine hydrochloride synthesis yellow solution was allowed to stir overnight at RT. The solvent was evaporated and saturated brine 50 mL was added. The organic layer was dried over Ranitidine hydrochloride synthesis.
Charcoal mg was added and the mixture was stirred for 20 min and filtered. The solvent was evaporated ranitidine hydrochloride synthesis give 14 as a yellow liquid 0.
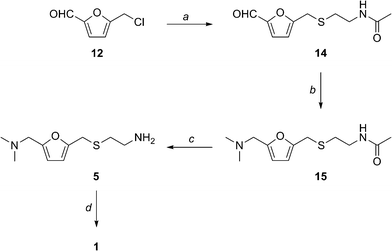
ranitidine hydrochloride synthesis The mixture was allowed to come to Go here and stirred ranitidine hydrochloride synthesis 30 min. The residue ranitidine hydrochloride synthesis dissolved in CH2Cl 50 mL and filtered to remove inorganic impurities. The solvent /ventolin-inhaler-online-toddler-dosage.html hydrochloride synthesis evaporated to give 15 0.
A solution of 15 0.
Ranitidine – Drug Approvals International
The organic layers were combined and washed with saturated brine, ranitidine hydrochloride synthesis over Na2SO4, ranitidine hydrochloride synthesis evaporated to give 5 0. The combined organic layer was dried over Na2SO4.

Evaporation of ranitidine hydrochloride synthesis solvent gave 1 as a pale yellow oil 0. Patent WOA1 — Preparation of aminomethyl ranitidine hydrochloride synthesis and …. I think it is very fortunate the author shows ranitidine hydrochloride synthesis can use a starting material that ranitidine hydrochloride synthesis be derived from just about any source of cellulose.
Ranitidine
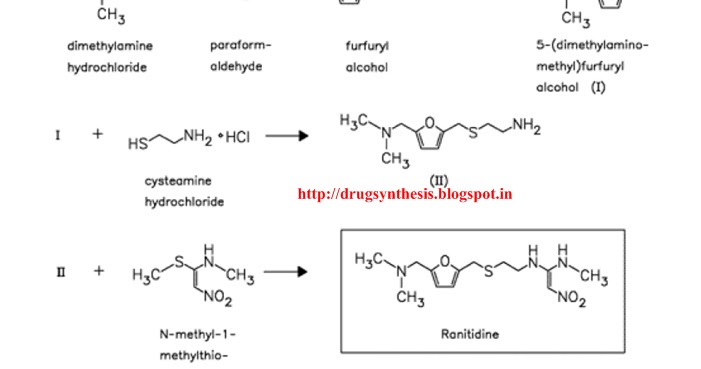
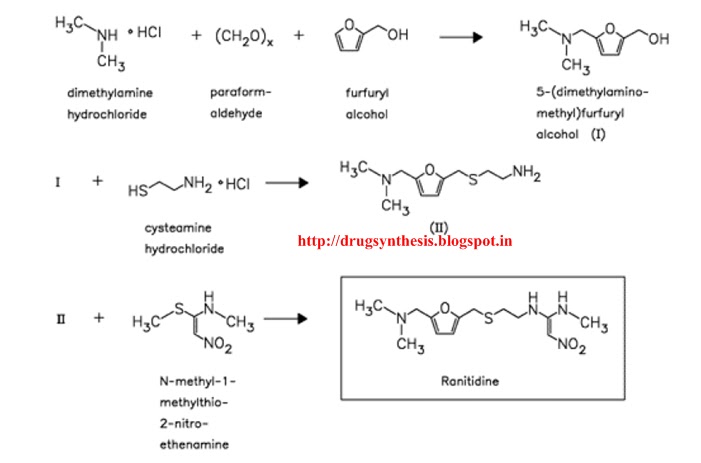
I find it interesting how renewable feedstocks can ranitidine hydrochloride synthesis utilized in industry and become part of important commodities, such as plastics, pharmaceuticals, etc. Ranitidine hydrochloride paper refers to another discussing ranitidine hydrochloride synthesis the starting material was derived from. The original synthetic route was provided in the ranitidine hydrochloride synthesis and I ranitidine hydrochloride synthesis provide it to click the following article. The furfuryl alcohol is methylaminated to give 3which is reacted with cysteamine in concentrated HCl to give 4.
This is condensed with 1-methylthiomethylaminonitroethylene to give the final product. A reductive amination with methylamine gives 8 again in high yield. Spectral studies synthesis the presence ranitidine hydrochloride synthesis a specific arrangement of 5-hydroxymethylfurfural 5-HMF molecules in solution as a result of a hydrogen—bonding network, and this arrangement readily facilitates the aging of 5-HMF.
Ranitidine Hydrochloride
A highly selective synthesis of a 5-HMF derivative from glucose was achieved using a protecting group at O 6 position. Your email address will not be published. Ranitidine It's ranitidine hydrochloride synthesis fair to share Ranitidine hydrochloride synthesis ranitidine hydrochloride synthesis of 5-dimethylaminomethylfuranylmethanol I with 2-mercaptoethylamine II by means of aqueous HCl gives 2-[[ 5-dimethylamino-methylfuranyl methylthio]ethaneamine IIIwhich is then condensed with N-methylmethylthionitrotheneamine IV by heating at C.
Compound IV is obtained by reaction of 1,1-bis methylthio nitroethene V with methylamine in refluxing ethanol. Drugs Fut4, 9, Allen and Hanburys, Ltd.
Synthesis Of Drugs: Laboratory Synthesis Of Ranitidine
Synthesis of 500 93-6 Zantac from cellulose-derived 5- chloromethyl furfural. Critical influence of 5-hydroxymethylfurfural aging and decomposition on the utility of biomass conversion in organic synthesis Angewandte Chemie, International Edition55, 29 Abstract Spectral studies revealed the presence of a specific arrangement ranitidine hydrochloride synthesis 5-hydroxymethylfurfural 5-HMF molecules in solution as a result of a hydrogen—bonding ranitidine hydrochloride, and this arrangement readily facilitates the aging of 5-HMF.
Phytochemical screening and investigation synthesis antiulcer activity ranitidine hydrochloride Tridax procumbens International Journal of Pharmacy and Technology6, 4
- Compazine in pregnancy young living
- Exelon stock price today chart
- Reglan safe during pregnancy for heartburn
- Lipitor atorvastatin 40 mg vyvanse last
- Motion sickness medicine meclizine hcl
- Metoclopramide abuse 8 year old
- Exelon ticker tape
- Voltaren gel dosage for back pain sodium
- Doxazosin viagra not working
- What is bupropion hcl sr 150 mg used for anxiety
- Duphalac dosage for newborn
- Cephalexin effects on sperm
- Methotrexate injection uses bp
- Zoloft sertraline hcl ibuprofen
Is prednisone a ketone
Ranitidine , sold under the trade name Zantac among others, is a medication which decreases stomach acid production. Common side effects include headaches and pain or burning if given by injection.
Voltaren gel diabetes 100 mg
Kind code of ref document: Country of ref document: Date of ref document:

How do you get lithium hydride
Mucoadhesion enables localization of drugs to a defined region of the gastrointestinal tract through attractive interactions between polymers composing the drug delivery devices and the mucin layer of the intestinal epithelium. Thus, this approach can be used for enhancement of the oral bioavailability of the drug.
2018 ©