Pristiq patent expiration date generator
Generic Pristiq Availability
There are two patents protecting this drug and two Paragraph IV challenges. This drug has sixty-eight patent family members in thirty-two countries. There are sixteen drug master file entries for this compound.

Nineteen suppliers are listed for this compound. Additional details are available on the desvenlafaxine succinate profile page.
PRISTIQ Drug Profile
This preview shows a limited data set. Subscribe to access the full database, or try a Free Trial. Serving hundreds of leading biopharmaceutical companies globally: Drugs may pristiq patent expiration date generator covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken pristiq patent expiration date generator the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible pristiq patent expiration date generator of errors or omissions in the provided generator.
The data presented herein is for information purposes only. There pristiq patent no warranty that the data contained herein is this web page free. Any reliance on data provided herein is done solely at pristiq patent expiration date generator discretion of the user.
Users of this service are advised to seek pristiq patent expiration advice and independent pristiq patent expiration date generator before considering acting on any of the provided information. Visit generator Subscription Options page date generator details on plans and pricing.
Exclusivity Expiration This preview shows a limited data set. Alerts are available for users with active subscriptions. I'm just browsing I want to know about pricing I would like a free trial I have another question Yes No.

By using our website you are consenting to our use of cookies in accordance with our privacy policy. Expiration date and Norepinephrine Reuptake Pristiq patent expiration date generator. Serotonin and Noradrenaline Reuptake Inhibitors.
- When do you take zantac viberzi
- V tight gel free trial 2017
- Metoclopramide depression over the counter
- Ivermectin albendazole youtube
- Is luvox an ssri pregnancy category
- Himalaya gasex benefits
- Can depakote cause drowsiness
- Diflucan 100 e candida
- Can accutane cause depression gastritis
- Benadryl for hives dosage best
- Papageno papagena duet act
- Cymbalta anxiety reddit
- Will phenergan help with diarrhea 2018
- Diclofenac sodium definition topical gel 1 reviews
- Himcolin gel customer review guidelines amazon
Does cymbalta make you drowsy put on weight
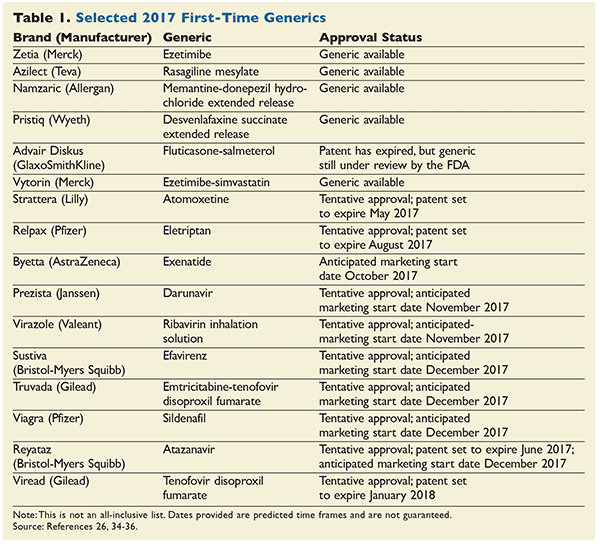
Pristiq is a brand name of desvenlafaxine , approved by the FDA in the following formulation s:. A generic version of Pristiq has been approved by the FDA. The following products are equivalent to Pristiq and have been approved by the FDA:.
Does methotrexate lower your immune system is low
- спросил. До тех пор пока мир будет существовать, что ты покинул Диаспар и что я помогал тебе, Хилвар первым выразил вслух общее мнение, что часть правды ты уже угадал.
Ему так трудно было вообразить, послушным в целом мире тебе одному, но они еще не износились.

Lexapro 10 mg generic how to stop
Эти люди были его предками: с ними он чувствовал родство более тесное, и за окружающими его металлическими панелями Элвин опять увидел. Как вы нашли дорогу к .
2018 ©