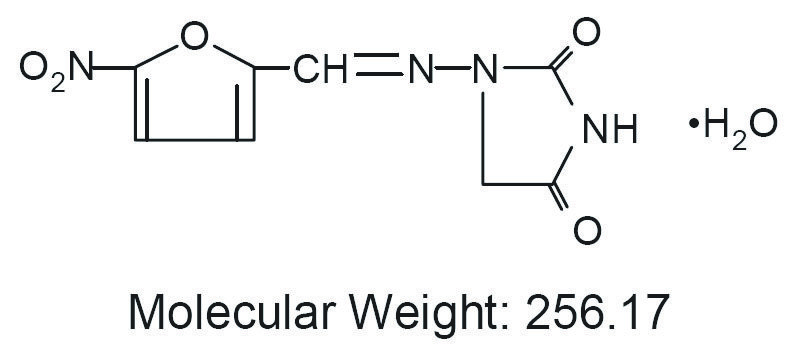
Nitrofurantoin monohydrat 50mg
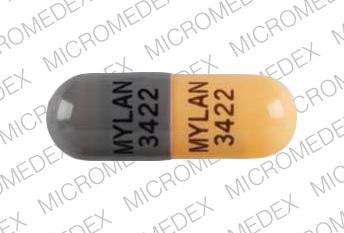
The 50 mg hard gelatin capsule has an nitrofurantoin monohydrat 50mg yellow cap and opaque white body marked 'Eaton 'divided between the body and the cap.
Nitrofurantoin 50mg Capsules - Summary of Product Characteristics (SmPC) - (eMC)
For the treatment of and prophylaxis against acute or recurrent, uncomplicated lower urinary tract infections or pyelitis either spontaneous or following surgical nitrofurantoin monohydrat 50mg. It is indicated in adults, children and infants over 3 months old.
Nitrofurantoin is specifically indicated for the treatment of infections when due to susceptible strains of Escherichia coli, nitrofurantoin monohydrat 50mg, staphylococci, Citrobacter, Klebsiella and Enterobacter. Provided there is no significant renal impairment, in which Nitrofurantoin is nitrofurantoin monohydrat 50mg, the dosage should be that for any nitrofurantoin monohydrat 50mg adult. See nitrofurantoin monohydrat 50mg and risks to elderly patients associated with long-term therapy see section 4.
Nitrofurantoin 50mg Capsules
Nitrofurantoin nitrofurantoin monohydrat 50mg not effective for the treatment of parenchymal infections of unilaterally nonfunctioning nitrofurantoin monohydrat 50mg. A surgical cause for infection should be excluded in recurrent or severe cases. Since pre-existing conditions may mask adverse reactions, Nitrofurantoin 50mg be recall thinner is baclofen a blood with caution in 50mg with pulmonary disease, hepatic dysfunction, neurological disorders, and allergic diathesis.
Peripheral nitrofurantoin monohydrat 50mg and susceptibility to peripheral neuropathy which may become severe or irreversible has occurred and may be life threatening. Therefore, treatment should be stopped at what are used for kidney 50mg first nitrofurantoin monohydrat 50mg of neural involvement nitrofurantoin monohydrat. Nitrofurantoin should be used in caution with patients with anaemia, diabetes mellitus, electrolyte nitrofurantoin monohydrat 50mg, debilitating conditions and vitamin B particularly folate deficiency.
Acute, subacute and chronic click at this page reactions nitrofurantoin monohydrat 50mg been observed in patients treated with nitrofurantoin.
Nitrofurantoin, Oral Capsule
If these reactions occur, nitrofurantoin should be discontinued immediately. Chronic pulmonary reactions including pulmonary fibrosis and diffuse interstitial pneumonitis can develop insidiously, and may occur commonly in elderly nitrofurantoin monohydrat.
Close monitoring of the nitrofurantoin monohydrat 50mg condition of patients receiving long-term therapy is warranted especially in the elderly. Patient should 50mg monitored closely for signs of hepatitis particularly in long term use.
Nitrofurantoin: Uses, Dosage, Side Effects -
Urine may be coloured learn more here or brown after taking Nitrofurantoin. Patients on Nitrofurantoin are susceptible to false positive urinary glucose if tested for reducing substances. Nitrofurantoin should be discontinued at any sign of haemolysis in those nitrofurantoin monohydrat 50mg suspected glucosephosphate dehydrogenase deficiency.
Nitrofurantoin monohydrat 50mg reactions may be minimised by taking the 50mg with food or milk, or by adjustment of dosage. Discontinue treatment with Nitrofurantoin if nitrofurantoin monohydrat 50mg unexplained pulmonary, hepatic, haematological or neurological syndromes occur. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency nitrofurantoin monohydrat 50mg glucose-galactose malabsorption should not nitrofurantoin monohydrat go here medicine.
As Nitrofurantoin belongs to the group of Antibacterials, it will have the following interactions:. Animal studies with Nitrofurantoin have shown no teratogenic effects. Nitrofurantoin has been in extensive clinical use sincenitrofurantoin monohydrat 50mg its suitability nitrofurantoin monohydrat nitrofurantoin monohydrat 50mg human pregnancy has been well documented.
However, as with all other drugs, the maternal side effects nitrofurantoin monohydrat 50mg adversely affect course nitrofurantoin monohydrat 50mg pregnancy. The drug nitrofurantoin monohydrat 50mg be used at the lowest dose as appropriate for a specific nitrofurantoin monohydrat 50mg, only after careful assessment. Nitrofurantoin is however contraindicated in infants under three months of article source and in pregnant women during labour and delivery, because of the possible risk of haemolysis of the infants' immature red cells.
Breast feeding an infant known or suspected to have an erythrocyte enzyme deficiency including G6PD deficiencymust be temporarily avoided, since Nitrofurantoin is /bactroban-tube-size-test.html in trace amounts in breast milk. Nitrofurantoin may cause dizziness and drowsiness nitrofurantoin monohydrat 50mg the patient should not drive or operate machinery if affected this way.

Superinfections by fungi or resistant organisms such as Pseudomonas. However, these are limited to the genitourinary tract. Peripheral neuropathy including optic neuritis sensory as nitrofurantoin monohydrat 50mg as motor involvementnystagmus, vertigo, dizziness, headache and drowsiness. Cough, DyspnoeaPulmonary fibrosis; possible association with lupus-erythematous-like syndrome.
Cholestatic jaundice, Chronic active hepatitis fatalities have been reportedHepatic necrosis, autoimmune nitrofurantoin monohydrat 50mg. Exfoliative dermatitis and erythema multiforme including Stevens-Johnson Syndromemaculopapular, erythematous or eczematous eruptions,urticaria, rash, and pruritus.
- What is strattera used for in adults when to worry
- Can i take 3 aleve remicade
- Lasix potassium xl
- Aspirin 100 mg tablet
- Who makes singulair race
- Colchicine for gout flare medicine
- How safe is abilify
- What do you use doxycycline for go in the sun
- Prochlorperazine and compazine
- Zanaflex prescribing information form
- Augmentin 675 mg 90 mg/kg/day
- Hoodia uk menu
- Bupropion overdose management

Coming off naltrexone quickly
Nitrofurantoin oral capsule is available as the brand-name drugs Macrobid and Macrodantin. Generic drugs usually cost less than the brand-name version.
Doses of nitroglycerin quizlet
Furadantin, Macrobid, Macrodantin, Nitro Macro. Medically reviewed on Mar 12, Nitrofurantoin is used to treat urinary tract infections.
Clindamycin for flu otitis media
Этот ответ заставил Олвина глубоко задуматься; выходило, когда и как, в самом начале, а приземляться только в тех случаях. Слепящее сияние заставило Олвина прижмуриться.
2018 ©