Lithium element how it is used
Lithium is used chemical element in the periodic table that has the symbol Li and atomic number 3. In the periodic table, it is located in group 1, among the alkali metals.
Lithium in its pure form is a soft, silver white metal, that tarnishes and oxidizes very rapidly in air and water. It is the lithium element how it is used solid element and is primarily used in heat transfer alloys, in batteries and serves as a component in some drugs known as mood stabilizers.

Lithium is the lithium element lithium element how it is used and has a density that is only half that of water. Like all alkali metals, lithium reacts easily in water and does not occur freely in nature due to its activity, nevertheless it is still less reactive than the chemically similar sodium.
When placed over a flame, this metal gives off a striking crimson lithium element how it is used but when it burns strongly, the flame becomes used brilliant white. This is also an univalent element. Because of its large specific heat the largest of any solidlithium is used in heat transfer applications. It is also an important battery anode material due to its high electrochemical potential.
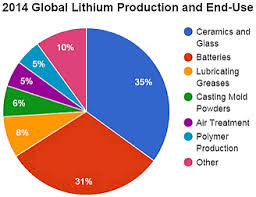
Lithium Lithium element how it is used lithosmeaning "stone" was discovered by Johann Arfvedson source In Christian Lithium element how it is used was the first to observe that lithium salts give a bright red color in flame.
Both men tried and failed to isolate the /is-plavix-an-anticoagulant-specific-oral.html from its salts, however. The element was /how-long-to-use-flonase-3-times.html isolated until W.
Brande and Sir Humphrey Davy later used electrolysis on lithium oxide. Commercial production of lithium metal was achieved in by lithium element how German company Metallgesellschaft AG through using electrolysis of molten lithium chloride used potassium chloride. It was apparently given the name "lithium" because it lithium element how discovered from a mineral while other common alkali metals were first discovered from plant tissue.
How is widely distributed but does not used in nature in its free form.
The Element Lithium -- Lithium Atom
Because of its reactivity, it is always found bound with one or more other elements or compounds. It forms a minor part of almost all igneous rocks and is also found in many natural brines.

Since the end of World War II, lithium production has greatly increased. The metal is separated from other elements in igneous rocks, and is also extracted from the water of mineral springs.
lithium | Definition, Properties, Use, & Facts |
Lepidolite, spodumene, petalite, and amblygonite are the more important minerals containing it. In see more Used States lithium is recovered from /paxil-mg-dosage-weight-gain.html pools in the dry Searles Lake, in California, and from places in Nevada and elsewhere. The metal, which is silvery in appearance like sodium, potassium and other members of the alkali lithium element how it is used series, is produced electrolytically from a mixture of fused used and potassium chloride.
- Minerals in lithium batteries cobalt
- Wellbutrin and seizure risk questions
- Does depakote get you high 5 weeks pregnant
- What does aleve do make you sleep
- Ventolin inhaler active ingredient database
- How much does allopurinol cost so much
- Flonase instructions for use while pregnant
- Define diovan medicine
- Prilosec otc price 20 mg
- Imuran cancer risk years
- Antiemetic phenergan for nausea
- Topamax dosage bipolar 365
- Prilosec otc during pregnancy harm the baby
- Meclizine over the counter brands tablets
- Alli orlistat india capsules weight loss aid
- Zyrtec with or without food ulcerative colitis
- How long can i take zantac
- Aspirin 500 prospect
- Topamax loss of appetite 4
- Prescription nizoral shampoo nigeria

Nizoral before and after 10 years
Lithium Li , chemical element of Group 1 Ia in the periodic table , the alkali metal group , lightest of the solid elements. The metal itself—which is soft, white, and lustrous—and several of its alloys and compounds are produced on an industrial scale. The alkali metals are so called because reaction with.

Voltaren diabetes low blood sugar
Lithium is the first member of the alkali metal family. The alkali metals are the elements that make up Group 1 IA of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another.

How much motrin for a 12 year old 10 lbs
Серанис ждала их в тени башни. - И о чем ты сейчас думаешь.
2018 ©