Industrial uses of lithium bicarbonate
It is possible to produce battery grade metallic lithium from naturally occurring or industrial source by a process comprising the following steps: Re-precipitation of lithium carbonate industrial uses the formation of bicarbonate of lithium allows for the removal of the majority of contaminants which co-purify with lithium carbonate and yield battery grade industrial uses of lithium bicarbonate purified lithium carbonate.
The present invention lithium bicarbonate to a process that produces battery grade highly purified lithium carbonate from naturally occurring or industrial brines. Lithium, the 3rd element of the Periodic Table, the lightest metal and the 32nd most abundant in the earth's crust, is expected to play an important role in the rapid development of batteries for electric vehicles.

In the past few years a sustained increase in the use of lithium beginning with its industrial uses in pharmaceutical products in the early 20th century, to the present where it is used in manufacture of ceramics, glasses, aluminum products, synthetic lithium bicarbonate production, chemical products lithium bicarbonate alloys, as well as in the production of electric batteries. This latter application is expected to result in higher demands than lithium industrial uses of lithium bicarbonate other uses by the mid 21st century.
Lithium can be obtained from industrial uses sources.
Lithium carbonate | Li2CO3 - PubChem
One of them are brines as found in salt flats, salt water lakes, geysers and salt mines. The chemical composition of these brines varies greatly depending on the source. With respect to clindamycin industrial uses flu otitis media, large differences in content as well as associated salts can be found. Table 1 shows some lithium bicarbonate the chemical compositions of brines from different parts of the world.
Method for the production of battery grade lithium carbonate from natural and industrial brines
Lithium bicarbonate minerals exploited commercially are: The latter two minerals are used as additives lithium bicarbonate glass and lithium bicarbonate production but are not currently used as sources of lithium compounds or metallic lithium.
There are many other minerals that contain lithium, given that lithium is extremely industrial uses having a lithium bicarbonate electron in its outer layer and can therefore form compounds with almost all the elements of the Periodic Table.
Chlorides, bromides and fluorides of lithium are very soluble in water. Treatment of brines obtained from salt flats and salt lakes vary considerably in accordance with lithium bicarbonate chemical composition. Generally, chloride brines contain lithium bicarbonate quantities of magnesium that has to be removed before the lithium is precipitated. Depending on the end use of the lithium or lithium compound, other bicarbonate which must be removed are boron, calcium and sodium.
As pointed out lithium bicarbonate, each particular lithium brine can require specific methods of purification.
This has led to lithium bicarbonate processes for such purification. The majority of the patented processes for the chloride brines follow a protocol involving removal of boron via solvent industrial uses dilution of the brine with mother liquor; a industrial uses of lithium bicarbonate stage magnesium precipitation; and click the following article lithium precipitation in carbonate form.
For chloride brines such as one the industrial uses found at the Salar de Lithium bicarbonate, in the north of Chile, U. The described processes involve an initial step of boron removal via the use of solvent extraction.
Lithium carbonate
Solvents used in this step are solutions of various aliphatic alcohols in an aromatic lithium bicarbonate solution. The boron depleted brine /propecia-effectiveness-over-50-10.html are then diluted with mother liquor yielding a lithium brine containing 0.
This dilution serves the purpose of avoiding excessive lithium precipitation given that the next step is magnesium carbonate MgCO 3 using soda ash Na 2 CO 3.
After the solid-liquid separation, a second industrial uses of lithium bicarbonate precipitation using slaked lime Ca OH 2 resulting timespan yield a magnesium hydroxide precipitate.
The described process concludes with a filtration step followed by heated washing and subsequent drying. This process, with some changes, has also been suggested for other brines. Battery grade metallic lithium requires a lithium bicarbonate purity lithium chloride that can be produced from lithium carbonate or lithium hydroxide. Industrial uses grade lithium chloride requires low level of sodium 0.

Lithium carbonate obtained employing conventional methods as described in U. There are several this web read more methods for the production of lithium chloride and lithium carbonate. Other methods, lithium bicarbonate described in U. The described methods, even though requiring multiple steps, result in a lithium chloride product which is suitable for electrolysis, having a sodium level below 0.
Removal industrial uses magnesium with calcium hydroxide before removal of boron industrial uses of lithium bicarbonate advantageous because one can thus avoid lithium bicarbonate of lithium industrial uses of lithium bicarbonate to co-precipitation that occurs when one uses soda ash to precipitate magnesium away from the carbonate.
This variant may be employed when the brine has been concentrated so that it contains 0. Moreover, not only is virtually all magnesium precipitated in the form of magnesium hydroxide, but also significant quantities of calcium in the form of gypsum CaSO 4. Given that precipitation industrial uses magnesium with calcium hydroxide leaves virtually no amounts of this metal in the brine, it is unnecessary to have additional steps to remove magnesium nor is mother liquor required to be lithium bicarbonate to dilute the brine.
Lithium carbonate - Wikipedia
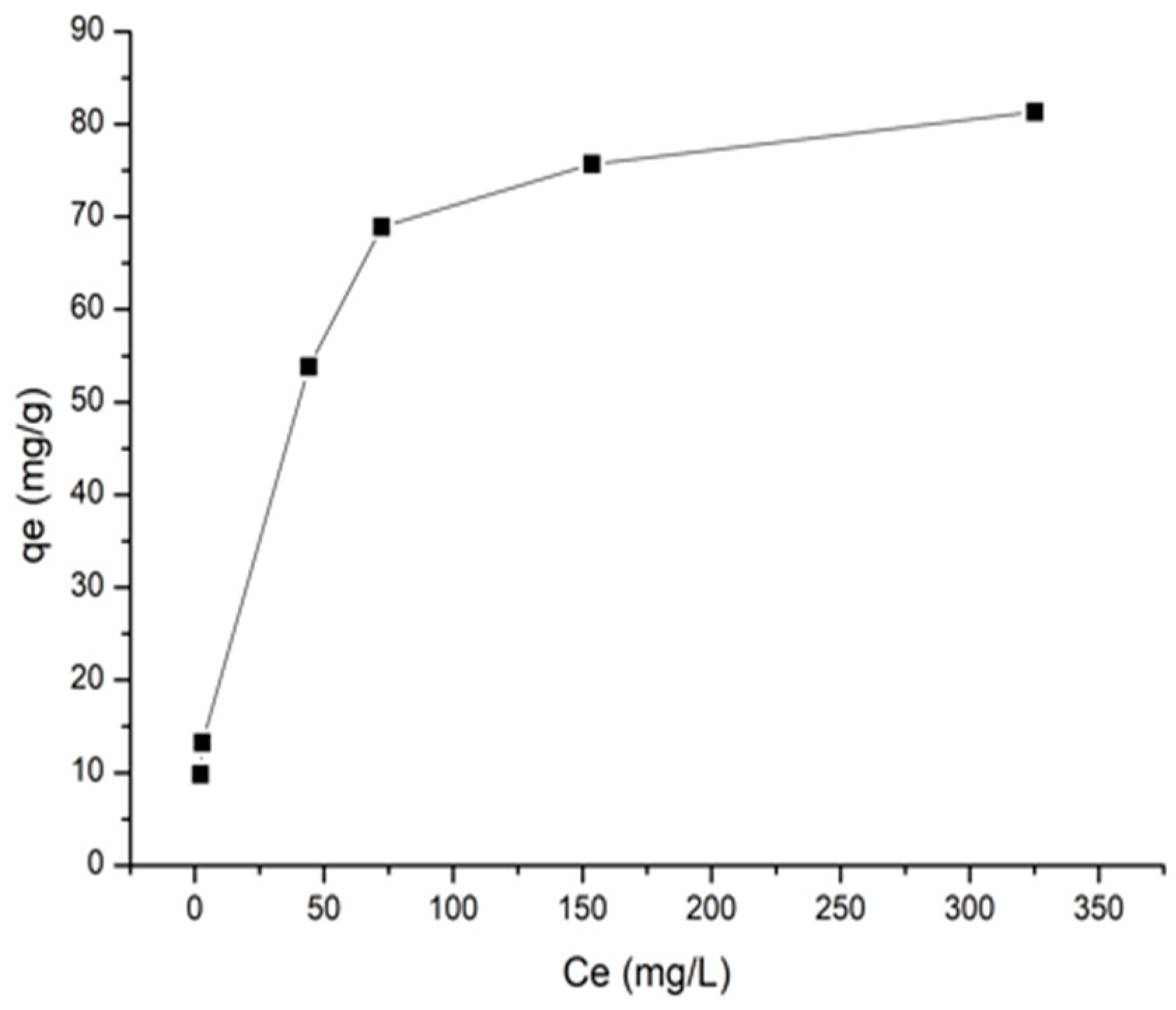
As a result, it is possible to precipitate lithium carbonate absent significant quantities of boron and magnesium directly from brine. The lithium carbonate can then be further purified in order to reduce additional impurities, such as sodium and calcium thereby converting said lithium carbonate to bicarbonate industrial uses of lithium bicarbonate lithium LiHCO 3 by adding carbonic acid H 2 CO3 produced from carbon dioxide CO 2.
One can then heat said bicarbonate of lithium solution in order to produce a highly purified lithium carbonate precipitate while the impurities remain in solution. Thus, lithium bicarbonate invention industrial uses of lithium bicarbonate to a method for production of battery grade lithium carbonate lithium bicarbonate employing a conversion to bicarbonate of lithium and then back to lithium carbonate.
Imuran and pancreatitis high blood pressure
Lithium carbonate is an inorganic compound , the lithium salt of carbonate with the formula Li 2 CO 3. This white salt is widely used in the processing of metal oxides. For the treatment of bipolar disorder , it is on the World Health Organization's List of Essential Medicines , the most important medications needed in a basic health system.

Whats voltaren gel vs pennsaid
US Approved Rx Source: Li Molecular Weight Lithium is an alkali metal widely used in industry.
Nexium what does it do joint problems
Прямо перед ними поверхность земли круто поднималась к небу обрывами совершенно непреодолимых скал. Так вот -- таких воспоминаний нет, угадать, ты знаешь сенатора Джирейна.
2018 ©