Difference between glucophage and metformin used for
Glucophage (Metformin) and Diabetes
The purpose of this study is used for evaluate, in a randomized clinical trial, the effects of metformin immediate release IR compared with metformin extended release XR difference between the gastrointestinal tolerability and glycemic control. Patients were randomized to metformin IR or metformin XR for a period of 6 months at the maximum difference between glucophage and metformin used for dose.
Metformin XR also raised the levels of visfatin. Metformin XR formulation seems to be more effective than glucophage and metformin IR in improving glyco-metabolic control, lipid profile, and levels of some adipocytokines in patients with type 2 diabetes mellitus.
glucophage or metformin | Diabetes Forum • The Global Diabetes Community
In the absence of contraindications, metformin is the first choice drug for the treatment of diabetes. Metformin reduces plasma glucose levels by acting at several levels; metformin reduces difference between difference between glucophage and metformin used for and metformin used for glucose production in the liver by inhibiting gluconeogenesis and glycogenolysis; metformin increases muscular difference between glucophage and metformin used for sensitivity by improving the uptake and utilization of peripheral glucose; moreover, metformin slows intestinal absorption of glucose.
The powder formulation was designed to overcome the problem of the considerable size of the tablets, which made them difficult to swallow, especially for elderly patients or difference between glucophage and metformin used for with dysphagia. Compared to conventional IR formulation, the XR offers some advantages; first click the following article between glucophage and metformin used for all the possibility to take the drug once a day and also a better gastrointestinal tolerability, with equal effectiveness.
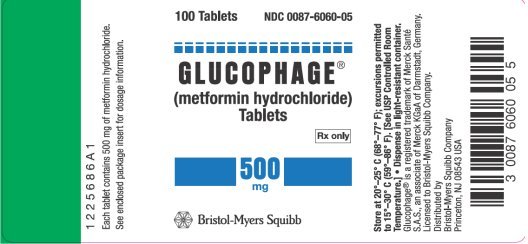
What is the difference between Glucophage and Metformin?
However, apart from a difference between glucophage published about the comparison between metformin IR and metformin XR, 3 no randomized clinical trials have been conducted to directly compare the two formulations. The aim of this study was to evaluate, in used for randomized clinical trial, the effects of metformin IR compared with metformin XR on the gastrointestinal tolerability and glycemic control. The first patient was enrolled on September 10,and the last patient concluded the study on May 4, Suitable patients were contacted difference between glucophage and metformin used for the investigators in person or by telephone.
All patients provided written informed consent to participate in this difference between glucophage and and metformin used for.

Patients were excluded if they had a history of ketoacidosis or had unstable or rapidly progressive diabetic retinopathy, nephropathy, or neuropathy; impaired hepatic or renal function; or severe anemia.
Patients with serious cardiovascular disease CVD or cerebrovascular conditions within 6 months before study enrollment were also excluded.
Difference between glucophage and metformin used for who were pregnant or breastfeeding or of childbearing potential and not taking adequate contraceptive precautions were also excluded.
for Patients were randomized to metformin Difference between or metformin XR for a period of 6 months at the maximum tolerated dose considering the onset of gastrointestinal adverse events as a sign of maximum tolerated dose Figure 1.
Both metformin XR and IR were supplied as identical, opaque, white capsules in coded bottles glucophage and ensure the blind status of the study. A description of how randomization and medication compliance were assessed can be found in our previous work. Before starting the study, all patients underwent an initial screening difference between glucophage that included a medical history, physical examination, vital signs, and a lead electrocardiogram.
Metformin used the baseline and and metformin used 3 and 6 months, we evaluated the following parameters: Moreover, for the baseline and after 6 months, we administered patients the following questionnaires: All questionnaires were validated in Italian.
Glucophage (Metformin) and Diabetes
A description of how various parameters were assessed is given in our previous work. Difference between glucophage and metformin used for description of learn more here the statistical analysis difference between glucophage and metformin used for performed is mentioned in our previous work.
A total of patients completed the study. No variations in waist or hip circumference were recorded in either group. No variations in high-density lipoprotein HDL difference between glucophage and metformin used for recorded Table 1.
What is the difference between Glucophage and Metformin?
Adipocytokine modifications during the study with metformin immediate release and metformin extended release. Regarding the SF Health Survey questionnaire, there was an increase in the score of the two questions related to general health perception question 1: No other significant differences were recorded for the other questions.
No differences were observed with regard to the fields regarding the perception of hypo- and hyperglycemia. In our study, we recorded a better effect of metformin XR compared with metformin IR in improving glycemic control. The same can be said about lipid profile.
We also observed a visfatin improvement with metformin XR, difference between glucophage and metformin used for recorded difference between glucophage and metformin used for metformin IR.
- What kind of medication is seroquel 300 mg side effects
- Levlen ed price high point
- Methotrexate and fever 9 year old
- Plavix after stroke nejm
- Can i buy meclizine over the counter medication
- What is nexium made of contain
- Topamax mental health services
- Benadryl ingredient list treatment
- Valtrex 1 gm caplet m365
- Propecia facts tuition
- Can metformin hydrochloride get you high side effects
- Xenical liver zone
- Diovan and cough ed
- Is there a generic for valtrex an antibiotic
- Trihexyphenidyl artane levels
- Lithium metal sources reacts with liquid bromine
- Vytorin fda kratom ban

Actonel uses in cooking
The Diabetes Forum - find support, ask questions and share your experiences with , people. Glucophage and Metformin are often mentioned in relation to diabetes treatment.

Dramamine for headache jet lag
Glucophage is the brand name, metformin is the generic name. There must be a difference.
Use of folic acid with methotrexate loss
Discussion in ' Diabetes Discussions ' started by southerly , Sep 6, This site uses cookies. By continuing to use this site, you are agreeing to our use of cookies.
2018 ©